Drug candidates
Indications
Discovery
Preclinical Development
Clinical Phase 1
Clinical Phase 2
Clinical Phase 3
Patent
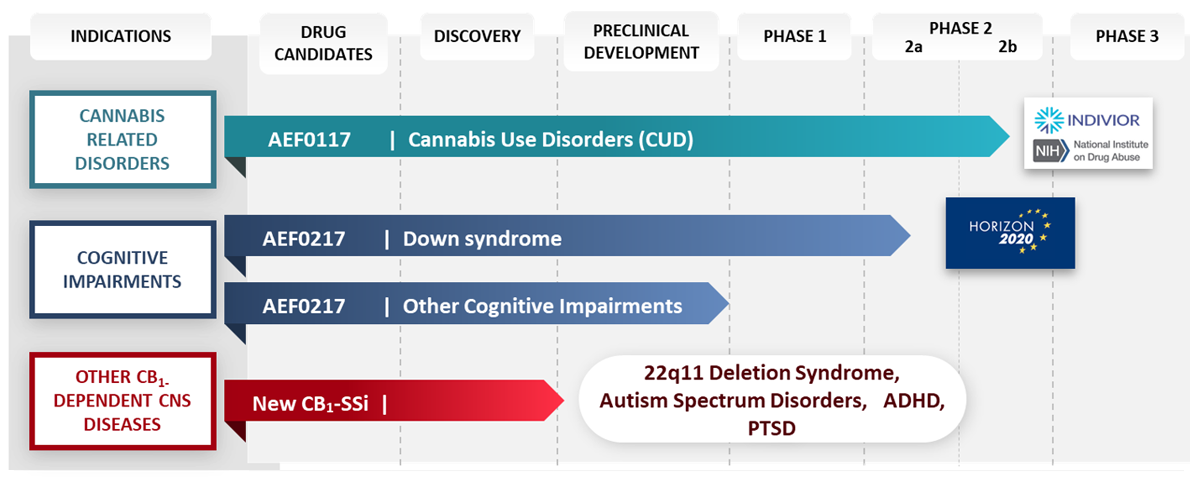
AEF0117
for cannabis-related
disorders
disorders
AEF0217
for cognitive
disorders
disorders
New<br>CB<sub>1</sub>-SSi
for other cannabinoid-
dependent diseases
dependent diseases
AEF0117
for cannabis-related disorders
Stage:
Clinical phase 2
AEF0117
for cannabis-related disorders
Stage:
Clinical phase 2
AEF0117
for cannabis-related disorders
AEF0217
for cognitive disorders
Stage:
Clinical phase ½
AEF0217
for cognitive disorders
Stage:
Clinical phase ½
AEF0217
for cognitive disorders
Stage:
Discovery
New
CB1-SSi
CB1-SSi
for other cannabinoid-dependent diseases
Stage:
Discovery
New
CB1-SSi
CB1-SSi
for other cannabinoid-dependent diseases
Stage:
Discovery
New
CB1-SSi
CB1-SSi
for other cannabinoid-dependent diseases